Kun for helsepersonell
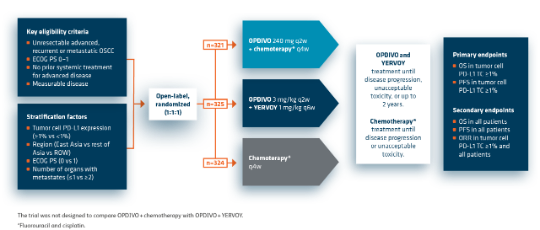
CheckMate 648: Study design1
CheckMate 648 compares OPDIVO + YERVOY or OPDIVO + chemotherapy with chemotherapy in patients with previously untreated, unresectable advanced, recurrent, or metastatic esophageal squamous-cell carcinoma. CheckMate 648 is the largest phase 3 trial in 1L metastatic OSCC.
- OPDIVO + chemotherapy*: In patients who received OPDIVO in combination with chemotherapy and in whom either fluorouracil and/or cisplatin were discontinued, other components of the treatment regimen were allowed to be continued
- OPDIVO + YERVOY: Patients who discontinued combination therapy because of an adverse reaction attributed YERVOY were permitted to continue with OPDIVO as a single agent
- Minimum follow-up: 28.8 months2
References:
- OPDIVO SmPC.
- Kato K, Doki Y, Chau I, et al. Nivolumab plus chemotherapy or ipilimumab versus chemotherapy in patients with advanced esophageal squamous cell carcinoma (CheckMate 648): 29-month follow-up from a randomized, open-label, phase III trial. Cancer Med. 2024; 13:e7235. doi:10.1002/cam4.7235.
7356-NO-2500069 / Developed 11.11.2025